Enantioselective direct Mannich-type reactions of 2-benzylpyridine N-oxides catalyzed by chiral bis(guanidino)iminophosphorane organosuperbase†
Abstract
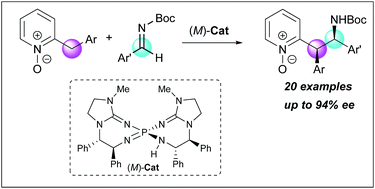
2-Benzylpyridine N-oxides possessing less acidic α-protons were utilized as pronucleophiles for the first time in enantioselective addition reactions under Brønsted base catalysis. A chiral bis(guanidino)iminophosphorane was able to overcome the inherent issue of low acidity of the pronucleophiles, establishing the diastereo- and enantioselective direct Mannich-type reaction with N-Boc imines. The control experiments indicated that the N-oxide moiety of the substrates played a critical role in achieving the high stereoselectivity.
- This article is part of the themed collection: 2021 Nobel Prize in Chemistry – Asymmetric Organocatalysis


 Please wait while we load your content...
Please wait while we load your content...