On the role of Ce in CO2 adsorption and activation over lanthanum species†
Abstract
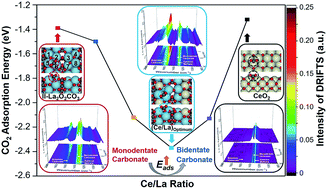
La2O3 exhibits good performance for various catalytic applications, such as oxidative coupling of methane (OCM) and dry reforming of methane (DRM), during which coke formation may lead to the deactivation of catalysts. Typically, the reaction between CO2 adsorbed on La2O3 and coke is the rate-determining step of the coke elimination process. This paper describes the influence of Ce addition on the CO2 adsorption and activation over La2O3. Combined with in situ and ex situ characterization and density functional theory (DFT) calculation, we show that Ce addition promotes the formation of bidentate carbonate on La2O3via tuning CO2 adsorption energy. In addition, Ce addition adjusts the ratio of bidentate/monodentate carbonate, and affects the ratio of hexagonal/monoclinic La2O2CO3 on the binary oxides. DRM is used as a probe reaction to examine the coke elimination performance of Ce–La binary oxide. It is found that when the Ce/La ratio reaches the optimal value (0.15), Ce–La binary oxide has the highest CO2 adsorption energy and predominantly promotes the formation of bidentate carbonate, and hence possesses the highest basicity above 700 °C and finally exhibits the best coke elimination performance.


 Please wait while we load your content...
Please wait while we load your content...