Diselenolane-mediated cellular uptake†
Abstract
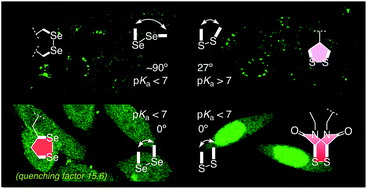
The emerging power of thiol-mediated uptake with strained disulfides called for a move from sulfur to selenium. We report that according to results with fluorescent model substrates, cellular uptake with 1,2-diselenolanes exceeds uptake with 1,2-dithiolanes and epidithiodiketopiperazines with regard to efficiency as well as intracellular localization. The diselenide analog of lipoic acid performs best. This 1,2-diselenolane delivers fluorophores efficiently to the cytosol of HeLa Kyoto cells, without detectable endosomal capture as with 1,2-dithiolanes or dominant escape into the nucleus as with epidithiodiketopiperazines. Diselenolane-mediated cytosolic delivery is non-toxic (MTT assay), sensitive to temperature but insensitive to inhibitors of endocytosis (chlorpromazine, methyl-β-cyclodextrin, wortmannin, cytochalasin B) and conventional thiol-mediated uptake (Ellman's reagent), and to serum. Selenophilicity, the extreme CSeSeC dihedral angle of 0° and the high but different acidity of primary and secondary selenols might all contribute to uptake. Thiol-exchange affinity chromatography is introduced as operational mimic of thiol-mediated uptake that provides, in combination with rate enhancement of DTT oxidation, direct experimental evidence for existence and nature of the involved selenosulfides.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...