Sublimable chloroquinolinate lanthanoid single-ion magnets deposited on ferromagnetic electrodes†
Abstract
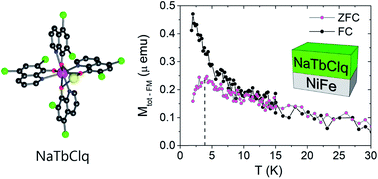
A new family of chloroquinolinate lanthanoid complexes of the formula A+[Ln(5,7Cl2q)4]−, with Ln = Y3+, Tb3+ and Dy3+ and A+ = Na+, NEt4+ and K0.5(NEt4)0.5+, is studied, both in bulk and as thin films. Several members of the family are found to present single-molecule magnetic behavior in bulk. Interestingly, the sodium salts can be sublimed under high vacuum conditions retaining their molecular structures and magnetic properties. These thermally stable compounds have been deposited on different substrates (Al2O3, Au and NiFe). The magnetic properties of these molecular films show the appearance of cusps in the zero-field cooled curves when they are deposited on permalloy (NiFe). This indicates a magnetic blocking caused by the interaction between the single-ion magnet and the ferromagnet. X-ray absorption spectroscopy confirms the formation of hybrid states at the molecule/metal interface.


 Please wait while we load your content...
Please wait while we load your content...