Investigation of spontaneous emulsification and its relevance in biodiesel synthesis†
Abstract
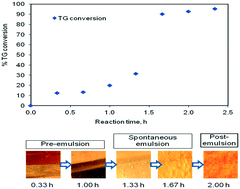
Transesterification of triglycerides (TGs) is associated with the coexistence of two sparingly miscible liquid phases. In this study, the reaction is carried out in a batch reactor with independent stirring of both the phases by maintaining a flat interface. An initial delay period followed by a sudden rise in reaction rate is observed. The S-shaped TG conversion curve thus represents an in situ change in the rate-controlling step during the course of reaction. The intrinsic reaction is reasonably fast and the progress of the reaction, in the initial period, is strongly influenced by the speed of agitation and the interfacial area. A spontaneous emulsion is observed as the reaction proceeds, resulting in a sudden rise in the interfacial area, and hence, the reaction rate. We observed that the change in the compositions of the individual phases was responsible for a decrease in the interfacial tension. At a particular concentration of the individual phases, a spontaneous emulsion is realized, beyond which, the reaction becomes kinetically controlled. During this period, the reaction rate is independent of agitation speed. The experimental results in both the pre- and post-emulsion period are explained using an appropriate model. We demonstrate that an appropriate selection of the initial composition of the two phases in a conventional batch reactor can conveniently eliminate the initial delay due to diffusional limitations.


 Please wait while we load your content...
Please wait while we load your content...