Enantioselective Nozaki–Hiyama–Kishi allylation-lactonization for the syntheses of 3-substituted phthalides†
Abstract
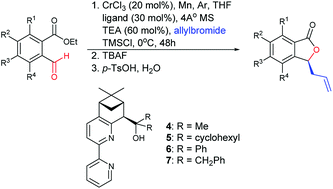
We report the synthesis of bipyridyl ligands and these ligands were applied in the chromium-catalyzed enantioselective Nozaki–Hiyama–Kishi allylation of substituted (2-ethoxycarbonyl)benzaldehydes and subsequently lactonized to synthesize phthalides with an optimal enantioselectivity of 99%. This approach was applied to synthesize (S)-cytosporone E at a 71% yield in three steps.

 Please wait while we load your content...
Please wait while we load your content...