Highly conjugated architectures and labile reaction intermediates from coupling between 10π electron-deficient heteroaromatics and sym-trihydroxy- or triamino-benzene derivatives†
Abstract
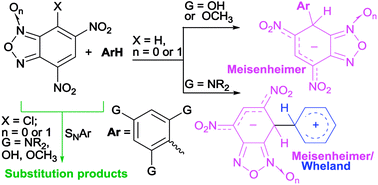
The C–C coupling reaction between neutral aromatic carbon nucleophiles such as 1,3,5-triamino- and 1,3,5-trihydroxy-benzene derivatives and 7-chloro-4,6-dinitrobenzofuroxan (Cl-DNBF) or 7-chloro-4,6-dinitrobenzofurazan (Cl-DNBZ) gave in high yield the corresponding conjugated structures, which are of potential interest in the materials field. When chlorine is absent on the electrophile, as in 4,6-dinitrobenzofurazan (DNBZ), the reaction with 1,3,5-triaminobenzene derivatives produces Wheland–Meisenheimer (WM) zwitterionic intermediates, intercepted and fully characterized via NMR at low temperature, whereas stable Meisenheimer complexes (M) were isolated in the reaction of phloroglucinol with 1,3,5-trimethoxybenzene. The latter also gave exclusively M complexes when reacted with 4,6-dinitrobenzofuroxan (DNBF), or 4,6-dinitrotetrazolopyridine (DNTP). The detection of WM or M intermediates can be rationalized by invoking the substituent stabilization ability of the positive charge in WM intermediates, on going from neutral carbon nucleophiles 1,3,5-triaminobenzenes to 1,3,5-trihydroxybenzene derivatives. Furthermore, variable-temperature NMR experiments on the M intermediates gave insights into the rotational barrier about the newly formed C–C bond.


 Please wait while we load your content...
Please wait while we load your content...