Solubility of sulfur dioxide in tetraglyme-NH4SCN ionic liquid: high absorption efficiency†
Abstract
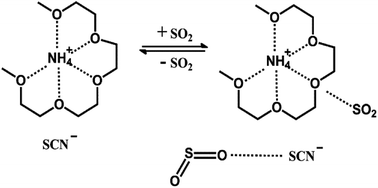
An easily prepared ionic liquid was synthesized by a one-step method and applied in SO2 absorption efficiently. The cation of the ionic liquid is a supramolecular structure consisting of NH+4 and tetraglyme, similar to the structure of NH+4 and crown ether, and the anion is selected as SCN−. The ionic liquid has good thermal stability. Under the conditions of 293 K and 1 bar, one mol ionic liquid can absorb 2.73 mol SO2, which is about 30% higher than tetraglyme. The absorption mechanism was characterized using IR and NMR. And the results confirmed that the interaction mechanism between SO2 and the ionic liquid is a physical interaction rather than a chemical interaction.


 Please wait while we load your content...
Please wait while we load your content...