Triterpenoids with α-glucosidase inhibitory activity and cytotoxic activity from the leaves of Akebia trifoliata†
Abstract
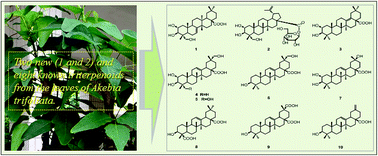
Ten pentacyclic triterpenoids including a new multiflorane triterpene acid, 2α,3β,23-trihydroxymultiflor-7-en-28-oic acid (1), and a new lupane triterpene monoglucoside named akebiaoside C (2), were obtained from the leaves of Akebia trifoliata. Their structures were elucidated by extensive spectroscopic analysis, and they were all isolated from the leaves of A. trifoliata for the first time. These compounds, except 4 and 5, showed in vitro α-glucosidase inhibitory activity much stronger than acarbose. Especially, 2, 3, 6, 8 and 10 displayed in vitro α-glucosidase inhibitory activity with IC50 values from 0.004 to 0.081 mM, which were close or even more potent than corosolic acid (IC50 0.06 mM). Triterpenoids 1, 8 and 10 were further revealed to show moderate in vitro cytotoxic activity against human tumor A549, HeLa and HepG2 cell lines, with IC50 values ranging from 26.5 to 51.9 μM. Compound 9 selectively showed in vitro cytotoxicity toward HeLa and HepG2 cell lines, with IC50 values of 81.49 and 73.47 μM, respectively. These findings provided new data to support that the leaves of A. trifoliata are a rich source in bioactive triterpenoids highly valuable to be developed for medicinal usage.


 Please wait while we load your content...
Please wait while we load your content...