1,2,3-Triazole–quinazolin-4(3H)-one conjugates: evolution of ergosterol inhibitor as anticandidal agent†
Abstract
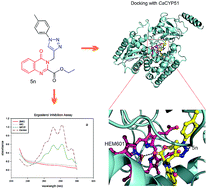
The present study describes the synthesis of 1,2,3-triazole–quinazolinone conjugates (5a–q) from ethyl 4-oxo-3-(prop-2-ynyl)-3,4-dihydroquinazoline-2-carboxylate and phenyl azide/substituted phenyl azides employing Cu(I) catalysed Huisgen 1,3-dipolar cycloaddition. The corresponding acids (6a–q) were obtained by hydrolysis of esters (5a–q) to study the effect of these functionalities on the biological activity. All synthesized compounds were screened for in vitro anticandidal evaluation against Candia albicans, Candida glabrata and Candida tropicalis strains. The results indicated that compound 5n showed potent anticandidal activity with IC50 in the range of 8.4 to 14.6 μg mL−1. Hemolytic activity using human red blood cells (hRBCs) and cytotoxicity by MTT assay on human embryonic kidney (HEK-293) cells revealed the non-toxic nature of the selected compounds. Growth kinetic study with compound 5n showed its fungicidal nature as no significant growth of Candida cells was observed even after 24 h. Cellular ergosterol content was determined in the presence of different concentrations of 5n to measure the activity of lanosterol 14α-demethylase indirectly. The results showed significant disruption of the ergosterol biosynthetic pathway through inhibition of lanosterol 14α-demethylase activity supported by docking studies (PDB: 5v5z). Overall, this study demonstrates the anticandidal potential of 5n which can serve as the lead for further structural optimization and SAR studies.


 Please wait while we load your content...
Please wait while we load your content...