A new family of fullerene derivatives: fullerene-curcumin conjugates for biological and photovoltaic applications†
Abstract
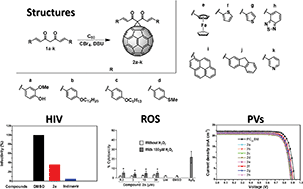
The synthesis and characterization of a family of [60]fullerocurcuminoids obtained via Bingel reactions is reported. The new C60 derivatives include curcumin and curcuminoids with a variety of end groups. Preliminary biological experiments show the potential activity of the compound containing a curcumin addend, which exhibits moderate anti-HIV-1 and radical scavenger properties, but no anti-cancer activity. In addition, the new fullerocurcuminoids exhibit HOMO/LUMO energy levels that are reasonably matched with those of perovskites and when they were tested in perovskite solar cells (PSCs) as the electron transporting material (ETM), photoconversion efficiencies ranging from 14.04–14.95% were obtained, whereas a value of 16.23% was obtained for [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) based devices.


 Please wait while we load your content...
Please wait while we load your content...