Investigation of the metabolic stability of olmutinib by validated LC-MS/MS: quantification in human plasma
Abstract
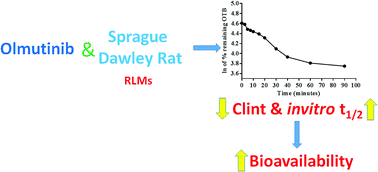
Olmutinib (OTB, Olita™) is an orally available third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI). It was developed by Boehringer Ingelheim and Hanmi Pharmaceutical Co. Ltd for the cure of non-small cell lung cancer (NSCLC). In May 2016, OTB was approved in South Korea for the treatment of patients suffering from metastatic or locally advanced EGFR T790M mutation-positive NSCLC. A LC-MS/MS methodology was validated for OTB quantification in human plasma. An extended application for this validated LC-MS/MS is OTB metabolic stability evaluation. Chromatographic separation of OTB and ponatinib (PNT, IS) was attained using a reversed phase with isocratic elution. The linearity of the developed LC-MS/MS method ranged from 5.00 to 500.00 ng mL−1 with r2 ≥ 0.9999 in human plasma. LOD and LOQ were 1.12 and 3.39 ng mL−1, respectively. The intra-day and inter-day precision and accuracy were 1.17 to 2.75% and 97.86 to 101.48%, respectively. The intrinsic clearance (CLint) was 2.71 mL min−1 kg−1 and the in vitro half-life (t1/2) was 48.80 min. A review of the literature revealed that there are no previous articles about the quantification of OTB in human plasma using LC-MS/MS or its metabolic stability assessment.


 Please wait while we load your content...
Please wait while we load your content...