A nitroalkane-based approach to one-pot three-component synthesis of isocryptolepine and its analogs with potent anti-cancer activities†
Abstract
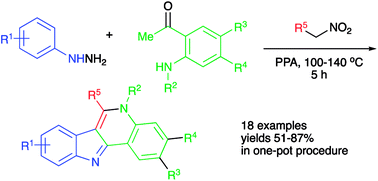
A second generation polyphosphoric acid-mediated one-pot three-component synthesis of indoloquinoline scaffold is developed. This improved version of the process involves electrophilically activated nitroalkanes for the installation of strategic C–C and C–N bonds and ring C assembly. This modification allows the elimination of unnecessary solvent change operations and all steps are carried out in a true, uninterrupted one-pot manner. A further improvement involves the possibility to install an ortho-amino group in situ. A synthetic application of this method is showcased by the concise synthesis of an isocryptolepine alkaloid and its synthetic analogs with potent anticancer activities.
- This article is part of the themed collection: Elegant Synthetic Routes to Indole Derivatives


 Please wait while we load your content...
Please wait while we load your content...