Synthesis and biological study of acridine-based imidazolium salts†
Abstract
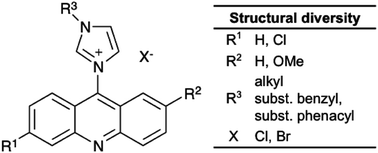
A new series of acridine based imidazolium salts was synthesized and evaluated for in vitro cytotoxicity against human cancer cell lines by an MTT assay. The synthesis applied a coupling of imidazoles with 9-chloroacridines, which originated from an Ullmann condensation of a 2-chloro-benzoic acid with an aniline. The target compounds were obtained in high yields. The DPPH assay indicated considerable antioxidant activity for target compounds with simple and short alkyl chains on the imidazole, while increasing chain length and the introduction of an additional π-electron system in most cases reduced the activity. All compounds exhibited low biotoxicity against non-cancerous cell lines, whereas a few compounds showed promising anticancer activity. Unlike for the reference drugs Tamoxifen and Paclitaxel, the anticancer activity of acridine imidazolium ions is specific for only selected cancer types. Reasonable fluorescent behaviour of the products provide potential for visualization of the distribution of active drugs in tissue.


 Please wait while we load your content...
Please wait while we load your content...