Inhibition of FAD-dependent lysine-specific demethylases by chiral polyamine analogues†
Abstract
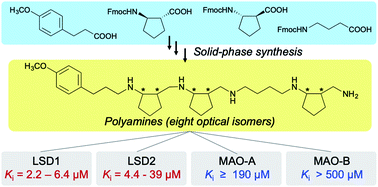
Lysine-specific demethylases 1 and 2 (LSD1 and LSD2) are flavoenzyme demethylases, and their inhibitors are considered as potential chemical tools and anticancer agents. Here we report polyamine-based inhibitors of LSD1 and LSD2. In the initial screening, partially constrained polyamine 2 which contains three trans-cyclopentane units with a total of six stereogenic centers, showed the most potent LSD1-inhibitory activity. We then prepared a set of optical isomers of 2 and evaluated their inhibitory activities toward LSD1, LSD2, monoamine oxidases A and B (MAO-A and MAO-B). Optical isomers of 2 showed LSD1-inhibitory activity with Ki values of 2.2 to 6.4 μM, and LSD2-inhibitory activity with Ki values of 4.4 to 39 μM; there was a general preference for LSD1 to LSD2. All of them showed weak to negligible inhibition of MAO-A and MAO-B. This selectivity seemed to reflect the differences in the size and shape of the catalytic cavity of target enzymes, and our strategy of employing a set of optical isomers appears to be an effective approach for exploring the structural features of this family of enzymes. Polyamine 9 showed most potent LSD1-inhibitory activity (Ki = 2.2 μM in vitro), and it also inhibited the proliferation of HL-60 cells (IC50 = 49 μM). On the other hand, 12 was the most potent inhibitors of LSD2 with in vitro Ki values of 4.4 μM.


 Please wait while we load your content...
Please wait while we load your content...