Nucleation and growth for magnesia inclusion in Fe–O–Mg melt
Abstract
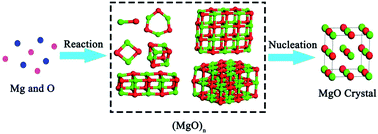
The crystallization process of magnesia in iron melt begins with nucleation, which determines the structure and size of magnesia inclusions. Thus, it is necessary to have a deep insight into the crystallization of magnesia by two-step nucleation mechanisms. In this work, the two-step nucleation method was used to investigate the behavior during the early stages of magnesia inclusions crystallization. A first principles method was applied to calculate the thermodynamic properties of magnesia crystal from various cluster structures for the formation of magnesia inclusions. Based on the numerical results, the nucleation mechanism of magnesia in liquid iron has been discussed. The magnesia clusters appear as the structural units for Mg-deoxidation reaction in the liquid iron, and the residual magnesia clusters are the reason for the supersaturation ratio or the excess oxygen for MgO formation in the liquid iron. Based on the comparison between Mg-deoxidation equilibrium experiments and numerical results, the previous experiments may be in a different thermodynamic state. The equilibrium reaction product should be not only magnesia clusters but also bulk-magnesia in those equilibrium experiments.


 Please wait while we load your content...
Please wait while we load your content...