Atomistic investigation of an Iowa Amyloid-β trimer in aqueous solution†
Abstract
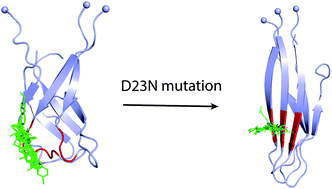
The self-assembly of Amyloid beta (Aβ) peptides are widely accepted to associate with Alzheimer's disease (AD) via several proposed mechanisms. Because Aβ oligomers exist in a complicated environment consisting of various forms of Aβ, including oligomers, protofibrils, and fibrils, their structure has not been well understood. The negatively charged residue D23 is one of the critical residues of the Aβ peptide as it is located in the central hydrophobic domain of the Aβ N-terminal and forms a salt-bridge D23-K28, which helps stabilize the loop domain. In the familial Iowa (D23N) mutant, the total net charge of Aβ oligomers decreases, resulting in the decrease of electrostatic repulsion between D23N Aβ monomers and thus the increase in their self-aggregation rate. In this work, the impact of the D23N mutation on 3Aβ11–40 trimer was characterized utilizing temperature replica exchange molecular dynamics (REMD) simulations. Our simulation reveals that D23N mutation significantly enhances the affinity between the constituting chains in the trimer, increases the β-content (especially in the sequence 21–23), and shifts the β-strand hydrophobic core from crossing arrangement to parallel arrangement, which is consistent with the increase in self-aggregation rate. Molecular docking indicates that the Aβ fibril-binding ligands bind to the D23N and WT forms at different poses. These compounds prefer to bind to the N-terminal β-strand of the D23N mutant trimer, while they mostly bind to the N-terminal loop region of the WT. It is important to take into account the difference in the binding of ligands to mutant and wild type Aβ peptides in designing efficient inhibitors for various types of AD.


 Please wait while we load your content...
Please wait while we load your content...