Transition metal-promoted hierarchical ETS-10 solid base for glycerol transesterification
Abstract
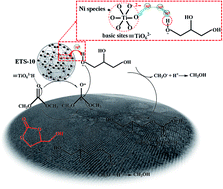
A transesterification reaction has been carried out over a transition metal modified hierarchical ETS-10 (METS-10) catalyst to synthesize glycerol carbonate (GC) from glycerol. The inherent Lewis basicity of ETS-10 favors oriented conversion of glycerol, and the hierarchical structure benefits exposure of more active sites, shortens the molecular diffusion path, suppresses the formation of coke in the micropores, and then enhances the catalytic reactivity and stability. Furthermore, the influence of transition metals (Fe, Co, Ni, Cu, Zn and Mn) on the basic sites of supports has been investigated in detail. It is found that basicity change of the catalyst depends on not only the cation size, nature and composition of the transition metal but also the zeolite structure and pore topology. Besides, the presence of a certain amount of Ni0 species from catalyst reduction has proved to play a critical role in strengthening the interaction of Lewis basic sites (TiO62−) with active glycerol hydroxyl groups. Finally, a 97.7% glycerol conversion and 97.1% GC yield have been obtained over Ni/METS-10, of which the high catalytic performance can be maintained after 8 runs.


 Please wait while we load your content...
Please wait while we load your content...