Chemical stability and interactions in a new antihypertensive mixture containing indapamide and dihydralazine using FT-IR, HPLC and LC-MS methods
Abstract
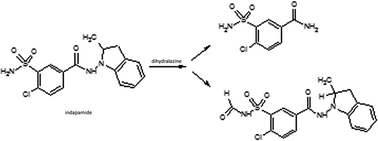
Indapamide and dihydralazine can be combined in fixed-dose formulations because of their complementary actions against hypertension. On the other hand, combined formulations present the problem of chemical interactions between the active ingredients, e.g. accelerated degradation of constituents or generation of quite new degradation products. Therefore, the main goal of the present study was to examine the chemical stability of indapamide and dihydralazine, as individuals and as a mixture, to detect potent interactions between both constituents, using FT-IR, HPLC and LC-MS methods. It was clearly shown that both drugs degraded more when they were in the mixture, i.e. indapamide was degraded more under high temperature/high humidity while dihydralazine was more sensitive to UV/VIS light. In solutions, indapamide was sensitive to strong acidic and strong alkaline conditions while dihydralazine degraded at pH ≥ 7. Generally, the process of degradation of indapamide and dihydralazine followed first order kinetics. The fastest degradation of both indapamide and dihydralazine was found at pH ≥ 10. Several degradation products of indapamide and dihydralazine were detected and identified by our LC-MS method. Interactions between both drugs were confirmed by detection of new degradation products of indapamide, i.e. 4-chloro-3-sulfamoylbenzamide and 4-chloro-3-(formylsulfamoyl)-N-(2-methyl-2,3-dihydro-1H-indol-1-yl)benzamide, only in the presence of dihydralazine.


 Please wait while we load your content...
Please wait while we load your content...