The streptazolin- and obscurolide-type metabolites from soil-derived Streptomyces alboniger YIM20533 and the mechanism of influence of γ-butyrolactone on the growth of Streptomyces by their non-enzymatic reaction biosynthesis†
Abstract
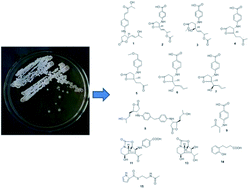
Eleven new compounds with streptazolin- and obscurolide-type skeletons were isolated from soil-derived Streptomyces alboniger obtained from Tibet, China. Two types of unprecedented skeletons of obscurolide dimer and an obscurolide-type compound with an aromatic polyketide of pentanone substituted at the benzene ring were determined by spectral data analysis. Compound 11 was the first evidence of two nitrogens in streptazolin-type structures. Compound 1 indicated an inhibitory effect on nitric oxide production in LPS-activated macrophages with an inhibition ratio of 51.7% at 50 μM, and on anticoagulant activity on platelet activating factor (PAF)-induced platelet aggregation with an inhibition ratio of 26.0 ± 9.1% at 200 μg mL−1. 11 had anti-acetylcholinesterase activity with an inhibition ratio of 27.2% at a concentration of 50 μM. Mechanistic aspects of the non-enzymatic reaction as well as a more detailed picture of the biosynthetic relationships of the streptazolin- and obscurolide-type metabolites are described. Acidic and basic conditions can inhibit the growth of Streptomyces, and γ-butyrolactones were found to be hormones controlling antibiotic production in Streptomyces. In the pH fermentation tests, acylation of γ-butyrolactones was successfully used to explain the mechanism of influence on the growth of Streptomyces.


 Please wait while we load your content...
Please wait while we load your content...