Total synthesis of kealiiquinone: the regio-controlled strategy for accessing its 1-methyl-4-arylbenzimidazolone core†
Abstract
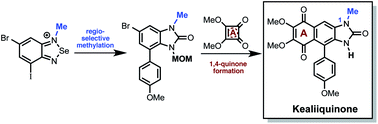
A practical, concise and straightforward total synthesis of kealiiquinone 1, a naphtho[2,3-d]imidazole alkaloid obtained from the Micronesian marine sponge Leucetta sp. was accomplished. The squaric acid chemistry to construct the 1,4-quinoid ring and the regioselective N-methylation through a benzo[c][1,2,5]selenadiazolium heterocycle are the key features in this report. The full details of the representative approaches involving the different attempted synthetic strategies are also presented. Finally a successful total synthesis of this complex secondary metabolite is described.


 Please wait while we load your content...
Please wait while we load your content...