Synthesis and performance evaluation of nanostructured NaFexCr1−X(SO4)2 cathode materials in sodium ion batteries (SIBs)
Abstract
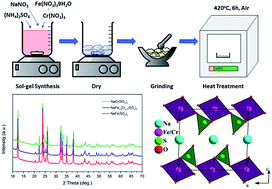
This research work focuses on the synthesis and performance evaluation of NaFexCr1−X(SO4)2 (X = 0, 0.8 and 1.0) cathode materials in sodium ion batteries (SIBs). The novel materials having a primary particle size of around 100–200 nm were synthesized through a sol–gel process by reacting stoichiometric amounts of the precursor materials. The structural analysis confirms the formation of crystalline, phase pure materials that adopt a monoclinic crystal structure. Thermal analysis indicates the superior thermal stability of NaFe0.8Cr0.2(SO4)2 when compared to NaFe(SO4)2 and NaCr(SO4)2. Galvanostatic charge/discharge analysis indicates that the intercalation/de-intercalation of a sodium ion (Na+) into/from NaFe(SO4)2 ensues at about 3.2 V due to the Fe2+/Fe3+ active redox couple. Moreover, ex situ XRD analysis confirms that the insertion/de-insertion of sodium into/from the host structure during charging/discharging is accompanied by a reversible single-phase reaction rather than a biphasic reaction. A similar sodium intercalation/de-intercalation mechanism has been noticed in NaFe0.8Cr0.2(SO4)2which has not been reported earlier. The galvanostatic measurements and X-ray photoelectron spectroscopy (XPS) analysis confirm that the Cr2+/Cr3+ redox couple is inactive in NaFexCr1−X(SO4)2 (X = 0, 0.8) and thus does not contribute to capacity augmentation. However, suitable carbon coating may lead to activation of the Cr2+/Cr3+ redox couple in these inactive materials.


 Please wait while we load your content...
Please wait while we load your content...