Photoinduced nucleophilic substitution of iodocubanes with arylthiolate and diphenylphosphanide ions. Experimental and computational approaches†
Abstract
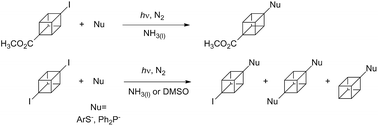
A new synthetic route to modify the cubane nucleus is reported here. Methyl-4-iodocubane-1-carboxylate (1) and 1,4-diiodocubane (2) were employed as reagents to react with arylthiolate and diphenylphosphanide ions under irradiation in liquid ammonia and dimethylsulphoxide. The reactions proceed to afford thioaryl- and diphenylphosphoryl- cubane derivatives in moderate to good yields. It is also found that the monosubstituted product with retention of the second iodine is an intermediate compound. Mechanistic aspects are supported by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...