Synthesis and anticancer studies of Michael adducts and Heck arylation products of sesquiterpene lactones, zaluzanin D and zaluzanin C from Vernonia arborea†
Abstract
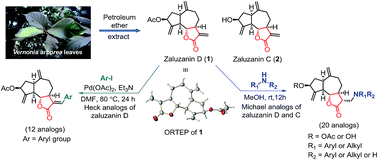
Sesquiterpene lactones containing α-methylene-γ-lactones, zaluzanin D 1 and zaluzanin C 2 were isolated from the leaves of Vernonia arborea. Several diverse Michael adducts (3–22) and Heck arylation analogs (23–34) of 1 have been synthesized by reacting with various amines and aryl iodides, respectively and were assayed for their in vitro anticancer activities against human breast cancer cell lines MCF7 and MDA-MB-231. Among all the synthesized analogs, Michael adducts 9 and 10 showed better anticancer activities as compared to 1. However, among these compounds, only 10 has minimal cytotoxic effect on normal breast epithelial MCF10A cells. Our detailed mechanistic studies reveal that compounds 9 and 10 execute their antiproliferative activity through induction of apoptosis and thereby inhibit the cancer cells proliferation and compound 10 could be a lead compound for designing potential anti-cancer compound.


 Please wait while we load your content...
Please wait while we load your content...