Theoretical research on excited-state intramolecular proton coupled charge transfer modulated by molecular structure
Abstract
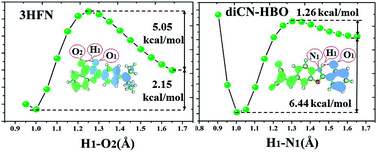
At the TD-B3LYP/TZVP/IEFPCM theory level, we have theoretically studied the excited-state intramolecular proton coupled charge transfer (ESIPCCT) process for both 4′-N,N-diethylamino-3-hydroxyflavone (3HFN) and 2-{[2-(2-hydroxyphenyl)benzo[d]oxazol-6-yl]methylene}malononitrile (diCN-HBO) molecules. Our calculated hydrogen bond lengths and angles sufficiently confirm that the intramolecular hydrogen bonds O1–H1⋯O2 and O1–H1⋯N1 formed at the S0 states of 3HFN and diCN-HBO should be significantly strengthened in the S1 state, which is further supported by the results obtained based on the analyses of infrared spectra shifts, molecular orbitals and charge density differences maps. The significant strengthening of intramolecular hydrogen bonds O1–H1⋯O2 and O1–H1⋯N1 upon photoexcitation should facilitate the ESIPCCT process of the two title molecules. The scanned potential energy curves and confirmed excited-state transition states for both 3HFN and diCN-HBO show that the proton can be easily transferred from O1 to O2 (N1 for diCN-HBO) through the strengthened intramolecular hydrogen bonds upon photoexcitation to the S1 state.


 Please wait while we load your content...
Please wait while we load your content...