Eco-friendly synthesis of ionic helical polymers and their chemical properties and reactivity†
Abstract
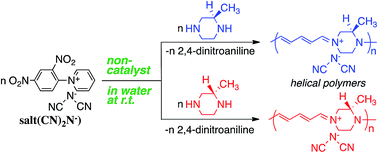
Reaction of N-(2,4-dinitrophenyl)pyridinium chloride (salt(Cl−)) with sodium dicyanamide (Na(CN)2N) resulted in anion exchange between Cl− and (CN)2N− to yield a new Zincke salt, salt((CN)2N−). Reactions of salt((CN)2N−) with piperazine, specifically (R)-(−)- or (S)-(+)-2-methylpiperazine under eco-friendly conditions, such as in aqueous solution, in the absence of a catalyst, and at room temperature, resulted in pyridinium ring opening to yield ionic high-molecular-weight polymers with 5-2,4-dienylideneammonium dicyanamide units or chiral 5-(2-methylpiperazinium)-2,4-dienylideneammonium dicyanamide units, namely, polymer(H;(CN)2N−), polymer(R-Me;(CN)2N−), and polymer(S-Me;(CN)2N−). UV-Vis measurements revealed that the π-conjugation system expanded along the polymer chain due to the orbital interaction between the electrons on the two nitrogen atoms of the piperazinium ring. Circular dichroism (CD) measurements revealed a helical conformation of the main chain in polymer(R-Me;(CN)2N−) and polymer(S-Me;(CN)2N−). The reaction of polymer(H;(CN)2N−) with p-phenylenediamine (PDA) caused recyclization of the 2,4-dienylideneammonium unit and resulted in depolymerization to yield N-(4-aminophenyl)pyridinium dicyanamide. Cyclic voltammetry analysis suggested that the polymers obtained in this study undergo electrochemical oxidation and reduction.


 Please wait while we load your content...
Please wait while we load your content...