Pyrolytic behavior of a zero-valent iron biochar composite and its Cu(ii) removal mechanism
Abstract
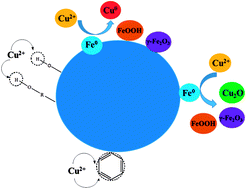
The reduction behavior of Fe3+ during the preparation of a zero-valent iron cocoanut biochar (ZBC8-3) by the carbothermic reduction method was analyzed. Fe3+ was first converted into Fe3O4, which was subsequently decomposed into FeO, and finally reduced to Fe0. A minor amount of γ-Fe2O3 was produced in the process. The isothermal thermodynamic data for the removal of Cu(II) over ZBC8-3 followed a Langmuir model. The Langmuir equation revealed a maximum removal capacity of 169.49 mg g−1 at pH = 5 for ZBC8-3. The removal of Cu(II) over ZBC8-3 fitted well to a pseudo-first-order equation, which suggested that the rate limiting step of the process was diffusion. The Cu(II) removal mechanism on ZBC8-3 involved the reduction of Cu(II) by Fe0 to produce Cu0 and Cu2O, while C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O–, and –O–H formed a complex with Cu(II).
C, C–O–, and –O–H formed a complex with Cu(II).


 Please wait while we load your content...
Please wait while we load your content...