Direct conversion of C6 sugars to methyl glycerate and glycolate in methanol†
Abstract
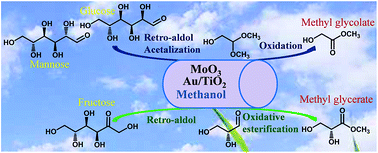
The present work deals with the one-pot conversion of C6 sugars to methyl glycerate and glycolate via a cascade of retro-aldol condensation and oxidation processes catalyzed by using MoO3 as the Lewis acid catalyst and Au/TiO2 as the oxidation catalyst in methanol. Methyl glycerate (MGLY) is the product of C6 ketose (fructose), while methyl glycolate (MG) is produced from C6 aldose (mannose, glucose). It is found that a good one-pot match between two reactive processes is the key to the production of MGLY and MG with high yield (27.6% MGLY and 39.2% MG). A separated retro-aldol condensation and oxidation process greatly decreases their yields, and even no MGLY can be obtained in this separated process. We attribute this to high instability of glyceraldehyde/glycolaldehyde and their different reaction pathways which mainly depend on whether acetalization of retro-aldol products (glyceraldehyde and glycolaldehyde) occurs with methanol or not. This result opens a new prospect on the accumulation of C3 products other than lactate from biomass-derived carbohydrates.


 Please wait while we load your content...
Please wait while we load your content...