Novel total syntheses of oxoaporphine alkaloids enabled by mild Cu-catalyzed tandem oxidation/aromatization of 1-Bn-DHIQs†
Abstract
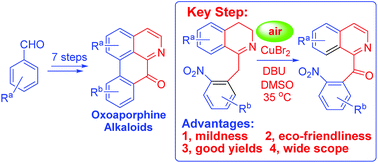
Novel total syntheses of oxoaporphine alkaloids such as liriodenine, dicentrinone, cassameridine, lysicamine, oxoglaucine and O-methylmoschatoline were developed. The key step of these total syntheses is Cu-catalyzed conversion of 1-benzyl-3,4-dihydro-isoquinolines (1-Bn-DHIQs) to 1-benzoyl-isoquinolines (1-Bz-IQs) via tandem oxidation/aromatization. This novel Cu-catalyzed conversion has been studied in detail, and was successfully used for constructing the 1-Bz-IQ core.


 Please wait while we load your content...
Please wait while we load your content...