Cerium and tin oxides anchored onto reduced graphene oxide for selective catalytic reduction of NO with NH3 at low temperatures
Abstract
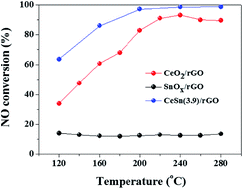
A series of cerium and tin oxides anchored on reduced graphene oxide (CeO2–SnOx/rGO) catalysts are synthesized using a hydrothermal method and their catalytic activities are investigated by selective catalytic reduction (SCR) of NO with NH3 in the temperature range of 120–280 °C. The results indicate that the CeO2–SnOx/rGO catalyst shows high SCR activity and high selectivity to N2 in the temperature range of 120–280 °C. The catalyst with a mass ratio of (Ce + Sn)/GO = 3.9 exhibits NO conversion of about 86% at 160 °C, above 97% NO conversion at temperatures of 200–280 °C and higher than 95% N2 selectivity at 120–280 °C. In addition, the catalyst presents a certain SO2 resistance. It is found that the highly dispersed CeO2 nanoparticles are deposited on the surface of rGO nanosheets, because of the incorporation of Sn4+ into the lattice of CeO2. The mesoporous structures of the CeO2–SnOx/rGO catalyst provides a large specific surface area and more active sites for facilitating the adsorption of reactant species, leading to high SCR activity. More importantly, the synergistic interaction between cerium and tin oxides is responsible for the excellent SCR activity, which results in a higher ratio of Ce3+/(Ce3+ + Ce4+), higher concentrations of surface chemisorbed oxygen and oxygen vacancies, more strong acid sites and stronger acid strength on the surface of the CeSn(3.9)/rGO catalyst.


 Please wait while we load your content...
Please wait while we load your content...