Deposition of methylammonium iodide via evaporation – combined kinetic and mass spectrometric study†
Abstract
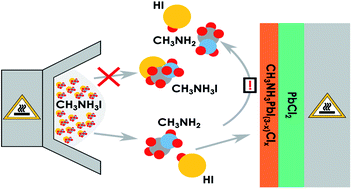
Methylammonium lead halide perovskites have recently emerged as a very attractive and versatile material for solar cell production. Several different perovskite fabrication methods can be used though most of them involve either spin coating, evaporation under high vacuum or a combination hereof. In this study we focus on thermal evaporation of methylammonium iodide (MAI), or more specifically, why this process, in terms of a physical vapour deposition, requires such a high deposition pressure to be successful. We use quartz crystal micro balance (QCM) measurements as well as mass spectrometry. The results indicate that MAI has a very low sticking especially if the substrate is held at elevated temperatures and is furthermore observed to evaporate with disproportionation into primarily CH3NH2 and HI. Even when PbCl2 is deposited on the QCM crystal, so that CH3NH3PbI(3−x)Clx perovskite can form, the MAI sticking remains low, possibly due to the requirement that both species be present on the film surface at the same time to form the perovskite. The results provide guidelines for designing a perovskite deposition chamber and additionally fundamental information about MAI evaporation.


 Please wait while we load your content...
Please wait while we load your content...