Synthesis of 3-(5-amino-1H-1,2,4-triazol-3-yl)propanamides and their tautomerism†
Abstract
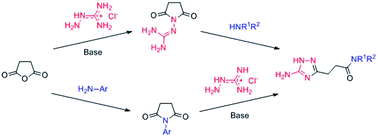
Two complementary pathways for the preparation of N-substituted 3-(5-amino-1H-1,2,4-triazol-3-yl)propanamides (5) were proposed and successfully realized in the synthesis of 20 representative examples. These methods use the same types of starting materials viz. succinic anhydride, aminoguanidine hydrochloride, and a variety of amines. The choice of the pathway and sequence of the introduction of reagents to the reaction depended on the amine nucleophilicity. The first pathway started with the preparation of N-guanidinosuccinimide, which then reacted with amines under microwave irradiation to afford 5. The desired products were successfully obtained in the reaction with aliphatic amines (primary and secondary) via a nucleophilic opening of the succinimide ring and the subsequent recyclization of the 1,2,4-triazole ring. This approach however failed when less nucleophilic aromatic amines were used. Therefore, an alternative pathway, with the initial preparation of N-arylsuccinimides and their subsequent reaction with aminoguanidine hydrochloride under microwave irradiation, was applied. The annular prototropic tautomerism in the prepared 1,2,4-triazoles 5 was studied using NMR spectroscopy and X-ray crystallography.


 Please wait while we load your content...
Please wait while we load your content...