Detection of SO2 derivatives using a new chalco-coumarin derivative in cationic micellar media: application to real samples†
Abstract
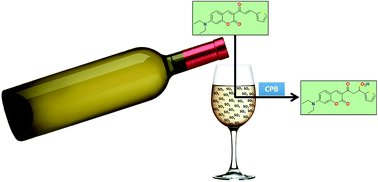
A new probe (E)-7-(diethylamino)-3-(3-(thiophen-2-yl)acryloyl)-2H-chromen-2-one (ChC16) was synthesized and studied as a turn-on fluorescent probe, based on a Michael addition mechanism for sensing SO2 derivatives, which is favored in the presence of cationic micellar media such as cetylpyridinium bromide (CPB). The probe showed high selectivity and sensitivity toward bisulfite over other anions and biothiols, including cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), with a detection limit of 240 nM. Moreover, the probe showed great potential for its practical application in the detection of bisulfite in real samples, such as dry white wine, and in bioimaging.


 Please wait while we load your content...
Please wait while we load your content...