8-Hydroxy-2-methylquinoline-modified H4SiW12O40: a reusable heterogeneous catalyst for acetal/ketal formation
Abstract
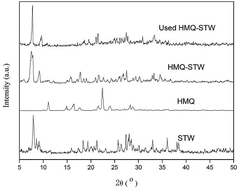
A heteropoly acid based organic hybrid heterogeneous catalyst, HMQ-STW, was prepared by combining 8-hydroxy-2-methylquinoline (HMQ) with Keggin-structured H4SiW12O40 (STW). The catalyst was characterized via elemental analysis, X-ray diffractometry (XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), thermogravimetric analysis (TG) and potentiometric titration analysis. The catalytic performance of the catalyst was assessed in the ketalization of ketones with glycol or 1,2-propylene glycol. Various reaction parameters, such as the glycol to cyclohexanone molar ratio, catalyst dosage, reaction temperature and time, were systematically examined. HMQ-STW exhibited a relatively high yield of corresponding ketal, with 100% selectivity under the optimized reaction conditions. Moreover, catalytic recycling tests demonstrated that the heterogeneous catalyst exhibited high potential for reusability, and it was revealed that the organic modifier HMQ plays an important role in the formation of a heterogeneous system and the improvement of structural stability. These results indicated that the HMQ-STW catalyst is a promising new type of heterogeneous acid catalyst for the ketalization of ketones.


 Please wait while we load your content...
Please wait while we load your content...