Aromatic heterocycle galectin-1 interactions for selective single-digit nM affinity ligands†
Abstract
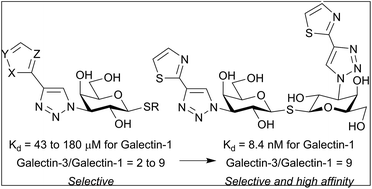
A series of 3-triazole-thiogalactosides and 3,3′-triazole-thiodigalactosides substituted with different five-membered heterocycles at the C-4 triazole position were found to have high selectivity for galectin-1. Initial studies on the 3-triazole-thiogalactosides indicated that five membered heterocycles in general gave increased affinity for galectin-1 and improved selectivity over galectin-3. The selectivity profile was similar for thiodigalactosides exemplified by 3,3′ substituted thien-3-yltriazole and thiazol-2-yltriazole, both having single-digit nM galectin-1 affinity and almost 10-fold galectin-1 selectivity. The binding interactions of a thiodigalactoside based galectin-1 inhibitor with two thien-3-yltriazole moieties were studied with X-ray crystallography. One of the thiophene moieties was positioned deeper into the pocket than previously reported phenyltriazoles and formed close contacts with Val31, Ser29, Gly124, and Asp123. The affinity and structural analysis thus revealed that steric and electronic optimization of five-membered aromatic heterocycle binding in a narrow galectin-1 subsite confers high affinity and selectivity.


 Please wait while we load your content...
Please wait while we load your content...