Characterization of NaxLi0.67+yNi0.33Mn0.67O2 as a positive electrode material for lithium-ion batteries†
Abstract
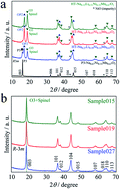
The relationship between the charge–discharge properties and crystal structure of NaxLi0.67+yNi0.33Mn0.67O2 (0.010 ≤ x ≤ 0.013, 0.16 ≤ y ≤ 0.20) has been investigated. Li/NaxLi0.67+yNi0.33Mn0.67O2 cells exhibit gradually sloping initial charge and discharge voltage–capacity curves. The initial charge capacity increased from 171 mA h g−1 for thermally-treated Na0.15Li0.51Ni0.33Mn0.67O2 to 226 mA h g−1 for Na0.010Li0.83Ni0.33Mn0.67O2 with an increase in the Li content. The initial maximum discharge capacity was 252 mA h g−1 in the case of Na0.010Li0.83Ni0.33Mn0.67O2 between 4.8 and 2.0 V at a fixed current density of 15 mA g−1 (0.06C) at 25 °C. The predominance of the spinel phase leads to the high initial discharge capacity of Na0.010Li0.83Ni0.33Mn0.67O2. This study shows that chemical lithiation using LiI is effective to improve the electrochemical properties.


 Please wait while we load your content...
Please wait while we load your content...