Cellulose triacetate synthesis via one-pot organocatalytic transesterification and delignification of pretreated bagasse†
Abstract
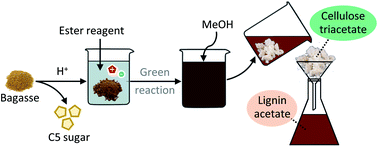
Cellulose triacetate was synthesised by the transesterification reaction of mild acid-pretreated lignocellulosic biomass with a stable acetylating reagent (isopropenyl acetate, IPA) in an ionic liquid (1-ethyl-3-methylimidazolium acetate, EmimOAc) which enabled the dissolution of lignocellulose as well as the organocatalytic reaction. The homogeneous acetylation of pretreated sugar-cane bagasse was carried out under mild conditions (80 °C, 30 min), and the subsequent reprecipitation processes led to enriched cellulose triacetate with a high degree of substitution (DS; 2.98) and glucose purity (∼90%) along with production of lignin acetate.


 Please wait while we load your content...
Please wait while we load your content...