Interfacial tension and CO2 diffusion coefficients for a CO2 + water and n-decane system at pressures of 10 to 160 bar†
Abstract
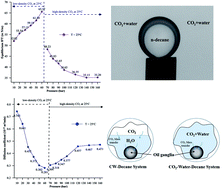
The objective of this study is to address the influence of different CO2 phases and degrees of CO2 saturation on the interfacial tension and the diffusion of CO2 into a hydrocarbon drop. Axisymmetric drop shape analysis on a pendant drop was used to carry out experiments in a pressure range of 10 to 160 bar and temperatures of 25 °C, 35 °C, and 45 °C, thus covering the gaseous, liquid, and supercritical phases of CO2. A numerical model that estimates the diffusion coefficient of CO2 in the hydrocarbon was developed. The IFT between the carbonated water and the hydrocarbon increases with pressure in the gaseous phase of CO2 and decreases in the liquid and supercritical CO2 phases. Interestingly, when the pressure was increased above 120 bar, the IFT did not change (decrease); this indicates that above this pressure, complete miscibility may not be achieved for this system, as indicated by the stable IFT. From the results, it can be concluded that the maximum IFT, maximum density decrease, and minimum diffusion coefficient occurred at pressures near to and below the phase change pressure of CO2 (64 bar at 25 °C and 74 bar at 35 °C and 45 °C). Both CO2–water–hydrocarbon and CW–hydrocarbon systems show the same trends; however, there were significant differences in the CO2 mass transfer rate and the concentration gradient.


 Please wait while we load your content...
Please wait while we load your content...