A highly selective fluorescent probe for detection of Cd2+ and HSO3− based on photochromic diarylethene with a triazole-bridged coumarin-quinoline group
Abstract
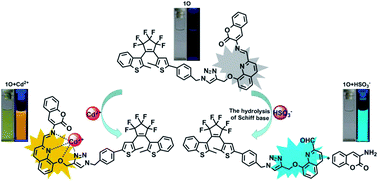
A novel photochromic diarylethene containing a quinoline-linked 3-aminocoumarin Schiff base unit (1O) was synthesized and used for the selective detection of Cd2+ and HSO3−. The synthesized probe exhibited a straightforward response for the selective detection of Cd2+. Its fluorescence emission red-shifted ∼126 nm and was enhanced 24.9 fold in the presence of Cd2+. Meanwhile, the fluorescence color of 1O changed from dark cyan to golden yellow. The binding stoichiometry between 1O and Cd2+ was determined to be 1 : 1. A molecular logic circuit with three inputs and one output was successfully constructed with its light and metal-responsive behaviors. In addition, 1O was able to selectively recognize HSO3− with a 135-fold enhanced fluorescence emission and a notable fluorescence color change from dark cyan to bright cyan. The 1H NMR and mass spectrometry analyses suggest that the HSO3− sensing of 1O is based on the hydrolysis of the Schiff base group of 1O.


 Please wait while we load your content...
Please wait while we load your content...