Pre-clinical pharmacokinetic-pharmacodynamic modelling and biodistribution studies of donepezil hydrochloride by a validated HPLC method†
Abstract
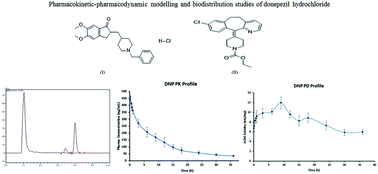
A simple, sensitive and robust HPLC–PDA assay was developed and validated for rapid determination of donepezil hydrochloride (DNP), a potent acetylcholinesterase inhibitor in rat plasma and tissues. All biological samples were prepared by the solid-phase extraction method using loratadine as an internal standard. Separation of the analytes was achieved on a Waters Nova-Pak C18 column (3.9 × 150 mm, 4 μm) using an isocratic mobile phase of acetonitrile and ammonium formate (pH 6.4; 0.01 M) (62 : 38% v/v) at a flow rate of 1 mL min−1. All validation parameter results were within the acceptable range described in the guidelines for bioanalytical method validation. The method showed linearity in the concentration range of 50–5000 ng mL−1 with LOD of 20 ng mL−1 and LLOQ of 50 ng mL−1. Moreover, the advantage of this method over previously published methods is the short analysis run time of 6 min in HPLC itself, alongside its application not only for plasma samples but also in tissues, with low LLOQ. The method was successfully applied for studying the compartmental pharmacokinetics, tissue distribution and pharmacodynamics. A two-compartmental micro model was statistically fitted for the assessment of pharmacokinetic parameters. The tissue distribution studies suggest that the kidneys, lungs and liver are the primarily responsible organs for metabolism and elimination of DNP. Pharmacodynamic studies were performed by measuring acetylcholinesterase inhibitory activity of DNP, which indicated that the pharmacokinetic and pharmacodynamic data are in correlation with each other.


 Please wait while we load your content...
Please wait while we load your content...