Activated release of bioactive aldehydes from their precursors embedded in electrospun poly(lactic acid) nonwovens
Abstract
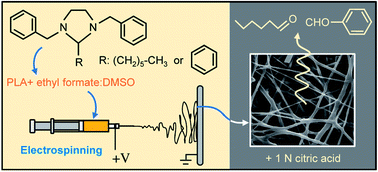
Hexanal and benzaldehyde are naturally-occurring aroma compounds from plants with enzyme-inhibition and antimicrobial properties. Although useful for food preservation applications, the end-use of these compounds can be challenging due to their volatility and susceptibility to oxidative degradation. In this study, stable precursors for benzaldehyde and hexanal were synthetized via reversible condensation reactions with N,N′-dibenzylethane-1,2-diamine. The molecular structures of the resulting 1,3-dibenzylethane-2-phenyl and 1,3-dibenzylethane-2-pentyl imidazolidines were confirmed by NMR analyses. The precursors were encapsulated in poly(lactic acid) fibers via electrospinning, using a 90 : 10 ethyl formate : dimethyl sulfoxide blend as a solvent. Triggered release of benzaldehyde and/or hexanal from the resulting active nonwovens was achieved by the addition of 1 N citric acid, which can be described using a pseudo first order kinetic equation involving rapid and slow release steps.


 Please wait while we load your content...
Please wait while we load your content...