Direct reaction between silicon and methanol over Cu-based catalysts: investigation of active species and regeneration of CuCl catalyst
Abstract
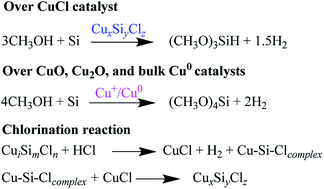
When a CuCl/Si mixture was pretreated at 200–240 °C in a N2 atmosphere, trimethoxysilane was predominantly formed in the direct reaction of silicon with methanol. When the pretreatment temperatures were raised to 260–340 °C, tetramethoxysilane was favorably formed. The CuxSiyClz species catalyzed the reaction between silicon and methanol to trimethoxysilane. Chlorination of the spent CuCl/Si mixture promoted the reaction between silicon and methanol to form both trimethoxysilane and tetramethoxysilane due to the recovery of the CuCl phase and the exposure of the metallic Cu0 phase. When Cu2O, CuO, and Cu0 were used as the catalysts, tetramethoxysilane was formed as the main product.


 Please wait while we load your content...
Please wait while we load your content...