Palladium-catalyzed tandem allylic substitution/cyclization and cascade hydrosilylated reduction: the influence of reaction parameters and hydrosilanes on the stereoselectivity†
Abstract
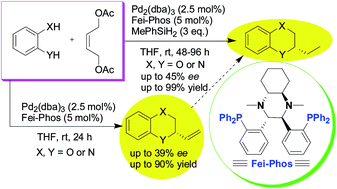
To shed light on the influence of reaction parameters on palladium-catalyzed tandem allylic alkylation in the presence of Fei-Phos (a chiral trans-1,2-diaminocyclohexane-derived phosphine ligand), the effect of different phosphine ligands, inorganic or organic bases, Brønsted acids, and other additives on the asymmetric palladium-catalysed alkylation of catechol with allylic diacetate was investigated. In this reaction, 2-vinyl-2,3-dihydro-benzo[1,4]dioxin products with promising enantioselectivity were achieved in good yields. In addition, a novel palladium-catalyzed three-component and one-pot allylic substitution/cyclization/reduction reaction assisted by methylphenylsilane was reported with good selectivity.


 Please wait while we load your content...
Please wait while we load your content...