Practical, mild and efficient electrophilic bromination of phenols by a new I(iii)-based reagent: the PIDA–AlBr3 system†
Abstract
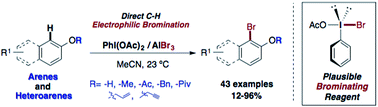
A practical electrophilic bromination procedure for phenols and phenol–ethers was developed under efficient and very mild reaction conditions. A broad scope of arenes was investigated, including the benzimidazole and carbazole core as well as analgesics such as naproxen and paracetamol. The new I(III)-based brominating reagent PhIOAcBr is operationally easy to prepare by mixing PIDA and AlBr3. Our DFT calculations suggest that this is likely the brominating active species, which is prepared in situ or isolated after centrifugation. Its stability at 4 °C after preparation was confirmed over a period of one month and no significant loss of its reactivity was observed. Additionally, the gram-scale bromination of 2-naphthol proceeds with excellent yields. Even for sterically hindered substrates, a moderately good reactivity is observed.


 Please wait while we load your content...
Please wait while we load your content...