Photodegradation of spent wash, a sugar industry waste, using vanadium-doped TiO2 nanoparticles†
Abstract
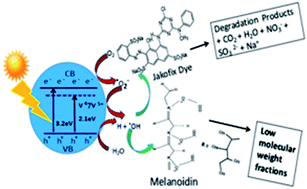
Waste from the sugar cane industry and alcohol distilleries is one of the sources of water pollution, and the degradation of this effluent is a very challenging task. Photocatalytic degradation can be an attractive alternative to conventional degradation processes. A vanadium-doped TiO2 (V-TiO2) photocatalyst for the degradation of spent wash and industrial dyes has been studied and reported here. V-doped TiO2 nanoparticles were prepared using a sol–gel method based on aqueous titanium peroxide with titanium isopropoxide as the Ti precursor and V2O5 as the V precursor. In order to observe the effect of the dopant on sol–gel behaviour, physicochemical and structural properties, the concentration of V was varied between 1.0% and 5% by weight. The crystallization temperature and time were optimized to obtain the required phase of V-TiO2. The physicochemical and structural characteristics of the V-doped TiO2 catalyst were determined using Brunauer–Emmett–Teller (BET), X-ray diffraction, FESEM, TEM, TG, FT-IR, Raman, PL and UV-visible spectroscopic techniques. UV-visible analysis showed a red shift in the absorption edge of TiO2 upon doping with V metal, which suggested an increase in the absorption of visible light due to a decrease in the effective band gap. The application potential of the V-TiO2 catalyst was studied via the degradation of sugar industry waste (spent wash) and Jakofix red dye (HE 8BN) under natural sunlight, as well as a standard artificial solar energy source (Xe lamp). The highest activity was observed for a 1% V-TiO2 photocatalyst for the degradation of spent wash and Jakofix red dye under natural sunlight. The degradation of coloured compounds in spent wash was monitored by gel permeation chromatography (GPC), which showed the degradation of high-molecular-weight compounds into low-molecular-weight fractions. The catalyst decomposed 90% of Jakofix red dye (HE 8BN) in 3.5 h and 65% of spent wash in 5 h under irradiation with natural sunlight, whereas Degussa P-25 TiO2 was only able to decompose 35% of the dye and 20% of spent wash under identical reaction conditions. A cycling stability test showed the high stability and reusability of the photocatalyst for degradation reactions, with a recovery of around 94–96%.


 Please wait while we load your content...
Please wait while we load your content...