Synthesis and biological evaluation of novel quinolone derivatives dual targeting histone deacetylase and tubulin polymerization as antiproliferative agents†
Abstract
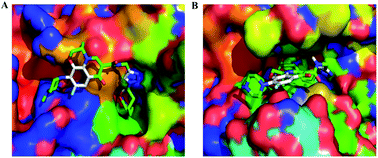
A strategy to develop chemotherapy agents by combining two complimentary chemo-active groups into a single molecule may have higher efficacy and fewer side effects than that of single-target drugs. In this article, we describe the synthesis and evaluation of a series of novel dual-acting levofloxacin–HDACi conjugates to target both histone deacetylase (HDAC) and tubulin polymerization. These bifunctional conjugates exhibited potent inhibitory activities against HDACs and tubulin polymerization. In docking analysis provides a structural basis for HDACs inhibition activities. Moreover, these conjugates showed selective anticancer activity that is more potent against MCF-7 compared to other four cancer cells A549, HepG2, PC-3, HeLa, but they had no toxicity toward normal cells.


 Please wait while we load your content...
Please wait while we load your content...