Diastereoselective construction of 4-indole substituted chromans bearing a ketal motif through a three-component Friedel–Crafts alkylation/ketalization sequence†
Abstract
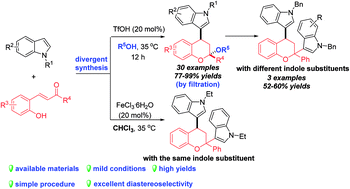
The first TfOH-catalyzed three-component Friedel–Crafts alkylation/ketalization sequence of indoles, alcohols and ortho-hydroxychalcones was developed to afford a wide range of 4-indole substituted chromans bearing a ketal motif in 77–99% yields. Notably, only a simple filtration was needed to purify them. By altering methanol to CHCl3, 2,4-bisindole substituted chroman with the same indole substituent at the C2 and C4 positions was afforded. Moreover, 2,4-bisindole substituted chromans with different indole substituents could be obtained by treatment of 4-indole monosubstituted chromans with another indole molecule.
- This article is part of the themed collection: Elegant Synthetic Routes to Indole Derivatives


 Please wait while we load your content...
Please wait while we load your content...