Diphenylpyrenylamine-functionalized polypeptides: secondary structures, aggregation-induced emission, and carbon nanotube dispersibility†
Abstract
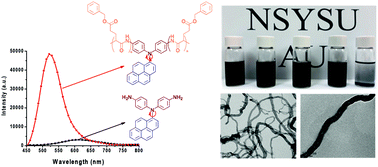
In this study we prepared—through ring-opening polymerization of γ-benzyl-L-glutamate N-carboxyanhydride (BLG-NCA) initiated by N,N-di(4-aminophenyl)-1-aminopyrene (pyrene-DPA-2NH2)—poly(γ-benzyl-L-glutamate) (PBLG) polymers with various degrees of polymerization (DP), each featuring a di(4-aminophenyl)pyrenylamine (DPA) luminophore on the main backbone. The secondary structures of these pyrene-DPA-PBLG polypeptides were investigated using Fourier transform infrared spectroscopy and wide-angle X-ray diffraction, revealing that the polypeptides with DPs of less than 19 were mixtures of α-helical and β-sheet conformations, whereas the α-helical structures were preferred for longer chains. Interestingly, pyrene-DPA-2NH2 exhibited weak photoluminescence (PL), yet the emission of the pyrene-DPA-PBLG polypeptides was 16-fold stronger, suggesting that attaching PBLG chains to pyrene-DPA-2NH2 turned on a radiative pathway for the non-fluorescent molecule. Furthermore, pyrene-DPA-2NH2 exhibited aggregation-caused quenching; in contrast, after incorporation into the PBLG segments with rigid-rod conformations, the resulting pyrene-DPA-PBLG polypeptides displayed aggregation-induced emission. Transmission electron microscopy revealed that mixing these polypeptides with multiwalled carbon nanotubes (MWCNTs) in DMF led to the formation of extremely dispersible pyrene-DPA-PBLG/MWCNT composites. The fabrication of MWCNT composites with such biocompatible polymers should lead to bio-inspired carbon nanostructures with useful biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...