The influence of CePO4 nanorods on the CO oxidation activity of Au/GdPO4-rods†
Abstract
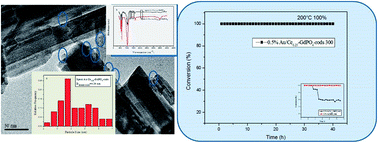
In this work, Au/GdPO4-rods were found to be good catalysts for CO oxidation with a low content of Au. The dopant of CePO4 could influence the activity of Au/GdPO4 due to the synergistic effect. GdPO4 and CePO4 nanorods were obtained by a hydrothermal process and the Au/GdPO4-rod and Au/Ce-GdPO4-rod catalysts were prepared by deposition–precipitation synthesis. The samples were extensively characterized by transmission electron microscopy (TEM), inductively coupled plasma (ICP), powder X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), ultraviolet-visible spectroscopy (UV-Vis), Fourier transform infrared spectroscopy (FT-IR), temperature programmed desorption (O2-TPD, CO-TPD, and CO2-TPD) and N2 adsorption–desorption. The results showed that Au/GdPO4 with a low Au content possessed good activity for CO oxidation. When the content of Ce is 25 at%, 0.5% Au/Ce-GdPO4-rods can convert CO completely at 65 °C, and the catalyst showed better high-temperature resistance than 0.5% Au/GdPO4-rods. 0.5% Au/Ce-GdPO4-rods also showed good stability at reaction temperatures of 55 and 65 °C with CO conversions of 90% and 100% after continuous operation for 12 h. They also showed no deactivation after 50 h at a relative high reaction temperature of 200 °C.


 Please wait while we load your content...
Please wait while we load your content...