One-pot sequential multicomponent reaction between in situ generated aldimines and succinaldehyde: facile synthesis of substituted pyrrole-3-carbaldehydes and applications towards medicinally important fused heterocycles†
Abstract
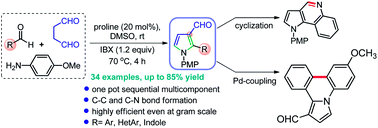
An efficient sequential multi-component method for the synthesis of N-arylpyrrole-3-carbaldehydes has been developed. This reaction involved a proline-catalyzed direct Mannich reaction-cyclization sequence between succinaldehyde and in situ generated Ar/HetAr/indolyl-imines, followed by IBX-mediated oxidative aromatization in one-pot operation. The practical utility of this procedure is shown at gram-scale and the synthesis of diverse bioactive fused heterocyclic scaffolds such as pyrroloquinoline, pyrrolo-oxadiazole, dihydro pyrroloquinoline, and pyrrolo-phenanthridine.


 Please wait while we load your content...
Please wait while we load your content...